*Long Post Alert!*
This is my second attempt at this post following the event of last Thursday : an event, so far as I can recall, unique at Vale! Towers. I started taking timed notes very early on in proceedings for reasons which will shortly become clear and I now feel able to relate the story in a relatively objective way.
I'm hoping to use the post to collate my notes into some sort of order. That may help me (and you!) figure out what happened and indicate what, if anything, beyond further water-changes I may need to do to get back to my original intentions for Sheba 4. I've still got tests in progress but I think I have enough facts and figures to make a substantial start. Perhaps the information in this post will be of value to the supplier of the fish in question.
For those who are first-time readers of this thread I'll sum up briefly where I'd got to by last Thursday morning. I'll try to stick to facts which seem relevant to current context.
Sheba 4 is a c75-litres long, shallow tank with a layer of JBL Manado black sand around 25mm deep. Temperature for cycling was set to around 25C. It is equipped with two airlines. It began cycling on 12th December last - fishlessly, using ammonia solution at a maximum in-tank concentration of c1.5mg/l and with the help of a small bag of mature media from a different tank. At the same time it received: Catappa and oak leaves ; decor (including wood, and red jaspar rock) ; and Protogen (infusoria pellets). During cycling the Total Ammonia concentration was allowed to fall to zero before recharging, as is my normal practice.
It was deemed cycled on January 10th and had two large water-changes to reduce the nitrate and phosphate resulting from both the cycling process and, in particular, the Protogen. Temperature adjusted to 24C. Addition of ammonia solution continued daily until the day before livestock was finally due to arrive. Three days later, on the 13th, some Helanthium
tenellus was introduced plus some JBL Kugeln root tabs ; on the 21st January a quantity of blackworms taken from my outside bath ; and on 22nd January a few silk 'plants'.
In December, and in anticipation, I had ordered twelve Corydoras duplicareus from an online supplier for delivery when the tank was ready. I won't name the supplier - at least until he has had a chance to read this, respond and maybe comment. In the meantime, of course, comments from Forum Members are most welcome. Once the tank was confirmed as cycled I had given the signal to the supplier that I was ready to receive. The supplier told me that he'd been having difficulties with the batch of
duplicareus in question and that there would be a delay of a week while the next batch finished their quarantine.
The original dispatch date (via APC) was scheduled for Tuesday 21st January with delivery the following day. In the event there was a further small delay of 24 hours so dispatch was on 22nd and the supplier warned me that there were eleven fish available to be sent.
The package arrived on Thursday 23rd January, at almost bang-on 1100hrs. As is my custom I left it to rest for half-an-hour or so before opening. Inside the outer cardboard carton was a polybox with a couple of small holes in its lid.

Inside the polybox there were two heat-packs - one taped to the lid and the other to the floor - which were just warm to the touch. The fishbag was wrapped in newspaper and supported horizontally by two balls of scrunched-up newspaper. The fish were double-bagged quite securely in around 2 litres of water. This pic shows the original disposition of the packing material - though by the time I took it I'd already emptied the bag.

What immediately grabbed my attention was that only two fish appeared to be showing obvious signs of movement ; and the bagwater had a faint-but-discernible milky cloudiness. It was at that point I started taking notes. When I opened the bag the smell was definitely not 'sweet'.
I tested the bagwater ...
- Temp : 17.9C
- Conductivity : 465uS
- pH : 7.06
- Ammonia-N : 1.69mg/l (= 2.05mg/l Total Ammonia)
- Unionised Ammonia (by calculation) : 0.007mg/l
- Nitrite-N : 12ppb (= 39.48mg/l NO2-N = 0.039mg/l nitrite)
I withdrew 2L of tankwater into a jug, floated the bag in the tank (keeping the top open with clothespegs), added ten drops of Seachem Prime and bubbled some air into its water using a turkey-baster (the latter was subsequently done after each partial exchange of bag/tank water). When temperatures were nearly equalised all the fish were comatose apart from two of the the smallest which were swimming actively. Three of the comatose fish were breathing sporadically.

I began a sequence of using a turkey-baster to withdraw small quantities of water every fifteen minutes or so, replacing it with an equal amount of water from the tank. I couldn't have used my drip-tube (because of the position of the tank) even if I had been able to locate it. I tracked the conductivity as a measure of progress.
Normally this method of acclimatisation continues until the conductivity of the bagwater roughly matches that of the tankwater. However the condition of the fish declined further. By 1700 the two active ones were swimming less strongly and from the others there were hardly any signs of life. Even though I'd got just over halfway to matching conductivities I netted the fish out of the bag into the tank in the hope that some may revive …

[You may be able to spot some blackworms at the front of the tank ; note that they had not buried their heads in the sand]
At 2300hrs the comatose fish had not moved. For some hours I hadn't been able to spot either of the two that had been relatively active.
Next morning, Friday, I removed the comatose fish (now confirmed dead) and found the other two amongst the decor, also dead.
Although somewhat despondent, I set about trying to find out what had happened. First I tested the tankwater more thoroughly in case I could identify something that might have contributed negatively overnight ...
- Temp : 23.8C
- Conductivity : 157.5uS
- pH : 6.36
- ORP : +139mV
- Dissolved Oxygen : 7.4mg/l
- Total Ammonia : 0
- Nitrite : 0
- Nitrate : 31.89mg/l [mostly a legacy from the Protogen, plus the cycling process]
- Phosphate : c10mg/l (calculated from x10 dilution) [mostly Protogen]
- Potassium : c7mg/l [Kugeln]
- Organics : "Low" [test done because bagwater 'frothed' slightly when oxygenated with turkey baster]
Nothing there which I would expect might account for fish deaths. Given the behaviour of the blackworms I applied a magnet to the substrate but nothing was attracted to it. Perhaps the sand is too tightly-packed, or its grains too small compared with the gravels they had been offered in previous indoor cultures. Later I did some further tests involving the substrate.
Further additions of ammonia showed that the cycle had not been compromised. There was a good population of infusoria on the oak and catappa leaves.
So I turned my attention to the small amount of bagwater which I had reserved.
I immersed a dipslide test and incubated it for 48hrs. It suggested that : the loading of aerobic bacteria was slight-to-moderate …

… and that the loading of yeasts and moulds was undetectable ...

There was, however, the same smell that I'd noticed when I'd first opened the bag of fish.
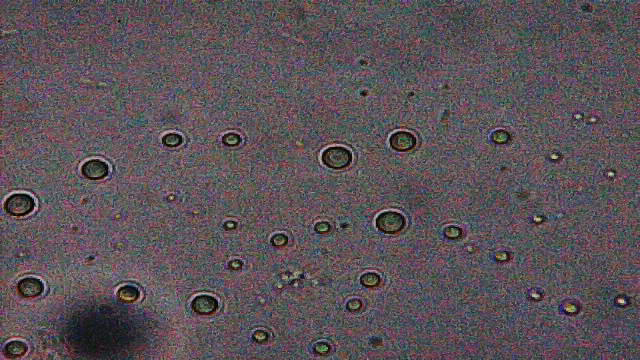
I looked closely at a sample of bagwater taken from the bottom of the bag (but didn't record what I saw due to an administrative error!). There was very much more activity than I expected in terms of micro-organisms whizzing around, seen at x200 ; at greater magnifications it was clear that this loading was very high. In particular, rod-shaped organisms were very numerous and their disposition in 'bunches' of clearly-dividing individuals suggested that they had been reproducing rapidly.
I took both skin and gill scrapes from two Corys ; a further skin scrape from another ; and a vent/gut scrape (I was a bit clumsy) from one of the first two. I videoed these and can edit and publish the recordings if required ; in the meantime I'm including some still images. The resolution of these images isn't great, I'm afraid.
This is from a Cory skin scrape. There were many of these 'bags' with seemingly non-motile organisms multiplying within …

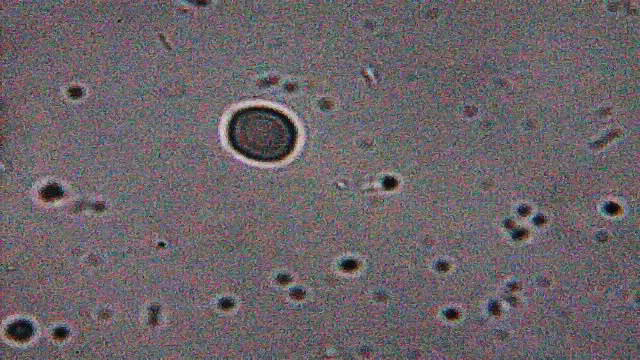
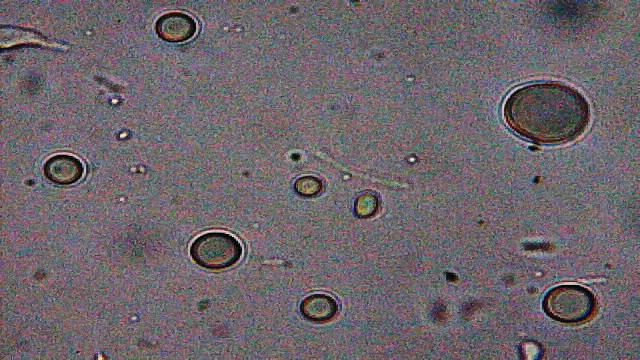
All samples contained examples of something that I'd not seen before and therefore raised suspicion. The magnifications (and light) available to me weren't good enough to resolve structure in any great detail but I could make out a multi-layered surface and activity within (5 images follow) ...
x600

x400
x400
x600
x600
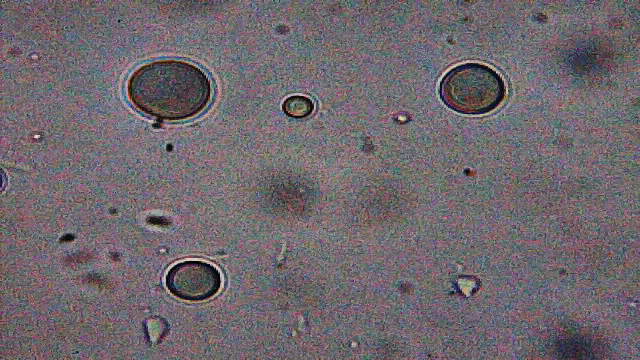
I spent quite some time online trying to look for images that were similar to these things. The closest I came to a reasonable match was Trichodina. That I wasn't able to resolve internal structure to the same degree as those with 'professional' instruments wasn't a surprise. There are a couple of characteristics of Trichodina that don't quite tally with what I saw : Trichodina are surrounded by cilia and are motile, while the vast majority of 'mine' seemed not to move (but I didn't use a well-slide, so they were trapped between slide and cover-slip) ; and Trichodina reproduce by fission and I couldn't see any of 'mine' that were obviously dividing in that way.
So that's by no means anywhere near conclusive but potentially a candidate. Reading further about these organisms and coupling that reading with the nature of the supplier's business (as derived from its name) could suggest cross-contamination as a possible source - assuming that they are Trichodina or something very similar.
Whichever agent cause the demise of the
duplicareus it may be very efficient at transfer to other fish [
see Addendum].
When looking at the bagwater earlier I had seen many 'rods' but none of the
presumed Trichodina-like creatures. So could the latter perish when not on a fish host for a short time, and therefore is the water itself in Sheba 4 likely now to be relatively safe?
I set up a test to try to find out, at the same time involving the substrate. I had three pairs of containers :

Each pair contained water taken from a different source. One of each pair had substrate added. Approximately ten Daphnia were put in each container and observed for 48 hours. Then two Sakura shrimp were put into each and observed for 48 hours.
Pair 1
The water, taken from my outside bath, had had no contact with either bagwater or tankwater. All Daphnia survived 48 hours in both containers. They survived another 68 hours while the shrimp were in. The shrimp survived 68 hours in both containers.
Pair 2
The water was from the 2 litres that had been withdrawn from the tank before the fish were introduced (aka: 'original tankwater'). After 24 hours all Daphnia were dead in the container without substrate ; in the other, four were alive. After 48 hours these latter were also dead. Could be a pH issue? All shrimp survived 68 hours.
Pair 3
This was water taken from the tank the morning after all fish were confirmed dead. After 24 hours, all Daphnia in the container without substrate were dead. In the other container, all were lying on the substrate with one or two showing signs of struggling life. After 48 hours they, too, were dead. pH issue again? All shrimp survived 68 hours.
I then took the container with bathwater-and-substrate and removed the animals to a temporary vessel. I emptied the container and washed the substrate very thoroughly in RO/DI. I had about 120mls of bagwater reserved in a closed jar. I looked at it with my microscope : it looked 'normal' in terms of visible micro-organisms. I put it into the container, topped it up with original tankwater and reintroduced the animals.
That was at 1600hrs yesterday. I checked just now and one shrimp has died - not altogether a surprise, perhaps, given the number of creatures in a small volume of water. The Daphnia are still alive in there.
Yesterday I also prepared a glass of water into which I put one of the silk plants (one of the two things whose names haven't yet been cleared). Later I shall transfer the animals to it to see how they react.
The other suspect whose name needs to be cleared is the filter sponge. Given that it was sold specifically as aquarium filter sponge it seems unlikely that it could be a contributor to the further decline of the
duplicareus once they were in the tank - but you never know! The same sponge is in Shebas 1 to 3 but as yet hasn't been tested by fish. I'll recruit some more shrimp later to try it out.
I tested the tankwater with a displide on the 28th and it came out of incubation this morning. It shows slight growth of aerobic bacteria and no yeast or moulds.
So that's where I'm up to at the moment. Any and all observations and/or speculations that anyone has will be welcome. I have my own opinions as to the contributory causes, of course, but have tried in this post to stick to presenting facts such as I have them.
Addendum
Given that the tank had been fishlessly-cycled the plan had been to stock all in one go, as is common practice, followed by close monitoring of water parameters until I was sure all was well. Accordingly, I introduced a group of Nannostomus beckfordi along with the duplicareus. I had been watching them in a local LFS whenever I had visted over a period of some weeks. They had looked perfectly healthy in the LFS during all that time. The morning after they had been put in the tank (following acclimatisation as described above) they were all dead, too! Skin scrapes revealed many of those Trichodina-type organisms.




























